Abstract
Introduction: Treatment options are limited in patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL). Combined therapy using agents with different mechanisms of action, such as an antibody‐drug conjugate (ADC) with an inhibitor of Bruton's tyrosine kinase (BTK), may improve therapeutic outcomes. Loncastuximab tesirine (loncastuximab tesirine-lpyl; Lonca) is an ADC comprising a humanized anti-CD19 monoclonal antibody conjugated to a pyrrolobenzodiazepine dimer toxin, approved for the treatment of patients with R/R DLBCL after ≥2 lines of prior systemic therapy. Ibrutinib, a small-molecule inhibitor of BTK, has shown antitumor activity - particularly as a combination treatment - in patients with R/R DLBCL (Depaus et al, abstract 2099, ASH Dec 5-8, 2020). Here, we present the results of a planned interim analysis in patients with DLBCL (irrespective of cell of origin), non-germinal center B-cell-like DLBCL (non-GCB DLBCL), and GCB DLBCL.
Methods: In this ongoing, Phase 2, open-label, single-arm study, patients aged ≥18 years with R/R DLBCL, measurable disease (per 2014 Lugano classification), and ECOG performance status 0-2 were enrolled. Patients were treated with Lonca 60 μg/kg once every 3 weeks for 2 cycles (patients who had a complete response [CR], partial response [PR], or stable disease were permitted to have an additional Lonca dose at Day 1 of Cycles 5, 6, 9, and 10), plus ibrutinib 560 mg/day taken orally for up to 1 year. The primary objective of the study was to evaluate the complete response rate (CRR) achieved with Lonca plus ibrutinib in patients with R/R non-GCB DLBCL (cell of origin, as determined by the investigator), assessed by central review. A Simon's 2-stage design was used in this study with a planned interim analysis conducted when the 22nd patient in the non-GCB DLBCL cohort had 2 tumor assessments. The objective of this planned interim analysis was to determine if CRR in the non-GCB DLBCL cohort warranted continuation of patient enrollment for study completion; if ≥6 patients achieved a CR the study was planned to proceed to the next stage. Thirteen patients with GCB DLBCL who met the defined cut-off for the interim analysis were also included in the dataset.
Results: As of April 21, 2021, 35, 22, and 13 patients with DLBCL overall, non-GCB DLBCL, and GCB DLBCL, respectively, were included in the planned interim analysis. In the overall DLBCL cohort, patients had a median age of 72 years (range 19-82) and had received a median of 3 prior therapies (range 1-6), including stem cell transplant. Patients in the overall DLBCL cohort received a median of 2 (range 1-6) cycles of Lonca and 4 (range 1-10) cycles of ibrutinib.
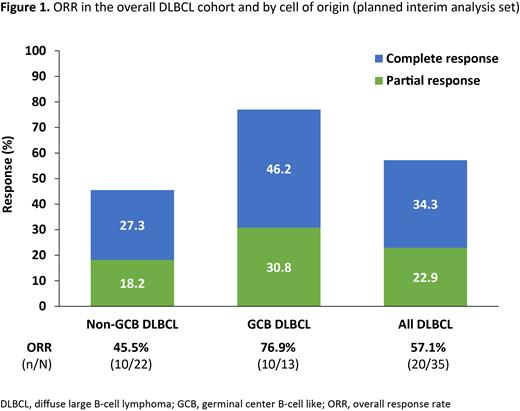
CRRs among the planned interim analysis population were 34.3% (12/35; 95% CI: 19.1-52.2), 27.3% (6/22; 95% CI: 10.7-50.2), and 46.2% (6/13; 95% CI: 19.2-74.9) in the overall DLBCL cohort, non-GCB DLBCL cohort, and GCB DLBCL cohort, respectively. ORR (CR + PR) was 57.1% (20/35; 95% CI: 39.4-73.7) in the overall DLBCL cohort, and 45.5% (10/22; 95% CI: 24.4-67.8) and 76.9% (10/13; 95% CI: 46.2-95.0) in the non-GCB DLBCL and GCB DLBCL cohorts, respectively (Figure 1). Median (95% CI) duration of response in the overall DLBCL cohort was 5.49 (5.49-not reached) months and was not reached in the non-GCB DLBCL or GCB DLBCL cohorts.
In the overall DLBCL cohort, non-GCB DLBCL cohort, and GCB DLBCL cohort, 32 (91.4%), 21 (95.5%), and 11 (84.6%) patients, respectively, had at least 1 treatment-emergent adverse event (TEAE). In total, 16 (45.7%) patients in the overall DLBCL cohort (15 [68.2%] with non-GCB DLBCL; 1 [7.7%] with GCB DLBCL) had Grade ≥3 TEAEs; the most common (≥10%) were neutropenia in 7 (20%) patients and thrombocytopenia in 4 (11.4%) patients. Overall, 17 (48.6%) patients had TEAEs leading to dose reduction, delay, or interruption. TEAEs leading to treatment discontinuation occurred in 8 (22.9%) patients.
Conclusions: At the doses tested, treatment with Lonca plus ibrutinib showed encouraging anti-tumor activity and a manageable safety profile in patients with R/R DLBCL. The study protocol will be amended to investigate whether Lonca given at each cycle in combination with ibrutinib improves efficacy outcomes in patients with R/R DLBCL.
Funding: ADC Therapeutics SA (NCT03684694).
Carlo-Stella: AstraZeneca: Honoraria; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Oncology: Honoraria; Incyte: Honoraria; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Karyopharm Therapeutics: Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Research Funding. Zinzani: Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sandoz: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; EUSA Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merck Sharp & Dohme: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kyowa Kirin: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Therapeutics Inc: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Verastem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen-Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Portola: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celltrion: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ImmuneDesign: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Janakiram: FATE Therapeutics: Research Funding; Takeda Pharmaceuticals: Research Funding; Kyowa Kirin Therapeutics: Honoraria; ADC Therapeutics: Research Funding. Dia: ADC Therapeutics America, Inc.: Current Employment, Current equity holder in publicly-traded company. He: ADC Therapeutics America, Inc.: Current Employment, Current equity holder in publicly-traded company; State University of New York Research Foundation: Current Employment. Ervin-Haynes: ADC Therapeutics America, Inc.: Current Employment, Current equity holder in publicly-traded company. Depaus: Celgene: Consultancy; Janssen: Consultancy; Novartis: Consultancy; Takeda: Consultancy.
Ibrutinib is not approved for DLBCL.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal